Real World Application of Freezing Point Depression
However if there is a possibility that the external temperature might go below 0C a coolant with a lower freezing point is needed. Freezing point depression is simply the process of LOWERING THE FREEZING POINT OF A LIQUID by adding a solute to it.

Freezing Point Depression Examples In Everyday Life Studiousguy
Thats why we use salt to melt ice on the road in the winter.

. Include a brief summary of the team discussion on individual results. The phenomenon of freezing point depression has many practical uses. And at a more practical level for chemists boiling point elevation and freezing point depression phenomena can be used to assess the molecularity of a solute and sometimes an accurate molecular mass.
Freezing point depression has interesting and useful applications. If you mix salt and ice in a bowl or bag the same process makes the ice colder which means it can be used for making ice cream. Road salting takes advantage of this effect to lower the freezing point of the ice it is placed on.
And this is a clear exploitation of the melting point depression phenomenon. Freezing point depression or boiling point elevation. Which of the following are real world examples of the application of the colligative properties.
Chemistry questions and answers. Write a 500-word reflection of the team discussion. Frozen fruit is not solid like ice because they contain sugar which prevents the water in the fruit from freezing.
This phenomenon is employed in the food industry ice cream and dessert making where saltsugar is added to a freezing mixture to make ice cream. Up to 24 cash back 1. Some insects make their own antifreeze glycol.
Learn why salt is used to make homemade ice cream and how you can make ice cream at home. Which of the following are real world examples of the application of the colligative properties. This project will reinforce the topics of freezing point depression molality and vant Hoff factor learned in a typical general chemistry course.
The increase of particles can still affect the freezing point of other solutions as a colligative property. When salt is put on an icy road the salt mixes with a small amount of liquid water to prevent melting ice from re-freezing. The radiator fluid in an automobile is a mixture of water and ethylene glycol.
Water and a solute which youll mix with the solvent. Lowering the freezing point of water. Some practical applications of freezing point depression are antifreeze in a radiator.
Water can be used as a coolant for car engines in warm climates. The freezing point The range was then adjusted to 000 and a depression varies for different pure solvents with second benzene sample is charged in and the Kf of water at 186⁰Cmol and benzene at ΔFp is read0882g of Nonane was dissolved in in 512⁰Cmol. -30 to -40 C -22 to -40 F.
The freezing point depression says that the freezing point of a mixture of two components is lower than that of individual components. The measurements of freezing point depression FPD are also used in the dairy industry to ensure that only the required amount of water is added to the milk. A very common example of this phenomenon in everyday life is salting of the roads in water.
The purpose of this activity is to further student understanding of a colligative property through a real-world practical issue. Real World Applications. As a result of freezing point depression radiators do not freeze up in winter unless it is extremely cold eg.
If the temperatures are below 18 o C calcium chloride is used instead of NaCl to melt the ice. Colligative properties have valuable applications such as the salting of roadways in cold-weather climates. Some important uses of freezing point depression are listed below.
Freezing Point Depression Lab Prep Key Points It is the number of solute particles not their identity which determines the magnitude of the boiling-point elevation and freezing point depression. And typically you use an ice-salt mixture when you churn the ice cream. Mixtures of ethylene glycol C 2 H 6 O 2 water and small amounts of other compounds are commonly used instead.
Uses of Freezing Point Depression. It involves a solvent eg. The freezing point depression could also be used for non water soluble solutions.
And as another example consider ice-cream. November 2014 Fall freezing point depression. What changes would you.
By applying salt to an icy road the melting allude of the ice is reduced and the ice will certainly melt more easily making driving safer. Salt is placed on roads to prevent snow from accumulating because it dissolves into solution with. The antifreeze helps prevent the water from freezing in the radiator and during the months when we salt the roads to help melt the snow.
Frozen fruit is not solid like ice because they contain sugar which prevents the water in the fruit from freezing. Freezing point depression or boiling point elevation. Discuss and compare your responses to the end-of-lab questions.
The stuff you make yourself with real cream and fruit and custard is much better than any ice cream you can buy. WHAT ARE SOME PRACTICAL APPLICATIONS OF FREEZING POINT DEPRESSION BOILING POINT ELEVATION AND VAPOR PRESSURE LOWERING. Salt is placed on roads to prevent snow from accumulating because it dissolves.
7 8 9. Properties based only on the number of solute particles and not their identity are known as colligative properties. The other aspects of the equation are the exact same except for the use of a different Kf constant for the new solvent as opposed to water.
Insects and natural antifreeze. In cold areas where the temperatures range from 0 o C to -15 o C sodium chloride is spread over the roads in order to lower the freezing point of water and prevent the build up of ice. In the laboratory cryoscopy is sometimes used to estimate the molecular mass of a new substance and this also relies on freezing point depression.
Ordinarily water freezes at 32F 0C but can you add salt to lower its freezing point to 20F -6C. Salt is placed on roads to decrease the freezing point of water preventing formation of ice in cold weather. Although temperatures may be freezing where these insects preside during the winter their cells do not freeze.
What are some practical applications of freezing point depression. See this old answer.

Freezing Point Depression Chemistry For Non Majors

The Science Of Ice Cream Freezing Point Depression Foodcrumbles
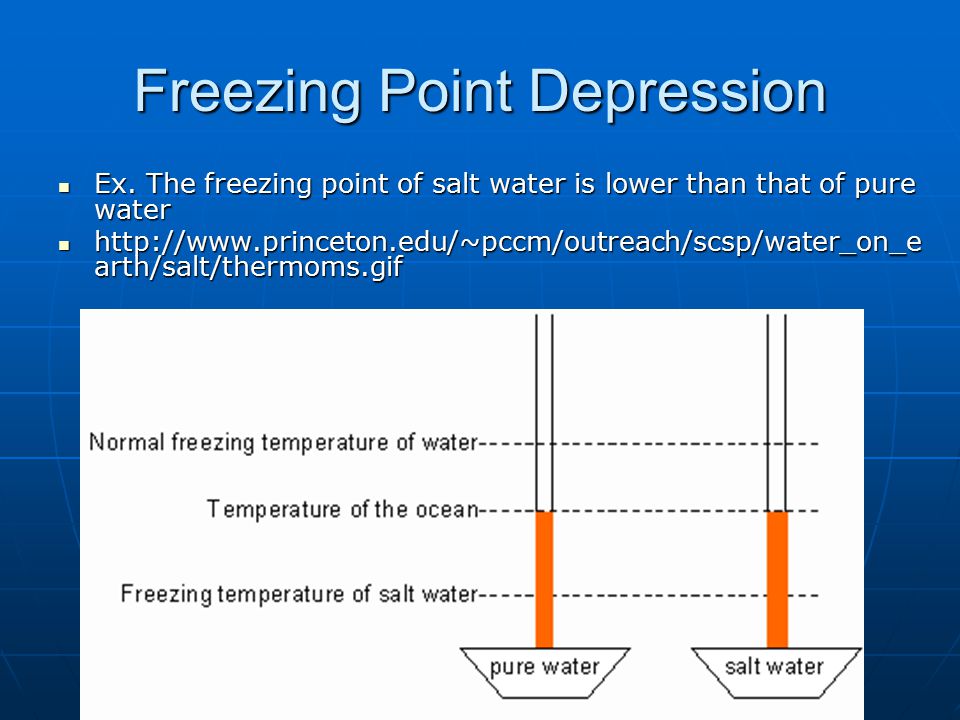
Determining The Molecular Mass By Freezing Point Depression Ppt Video Online Download

Freezing Point Depression Examples In Everyday Life Studiousguy

Freezing Point Depression With Example Problem Youtube

Change In Freezing Point Common Applications Of Freezing Point Depression Propylene Glycol Ethylene Glycol Deadly To Small Animals Ppt Download
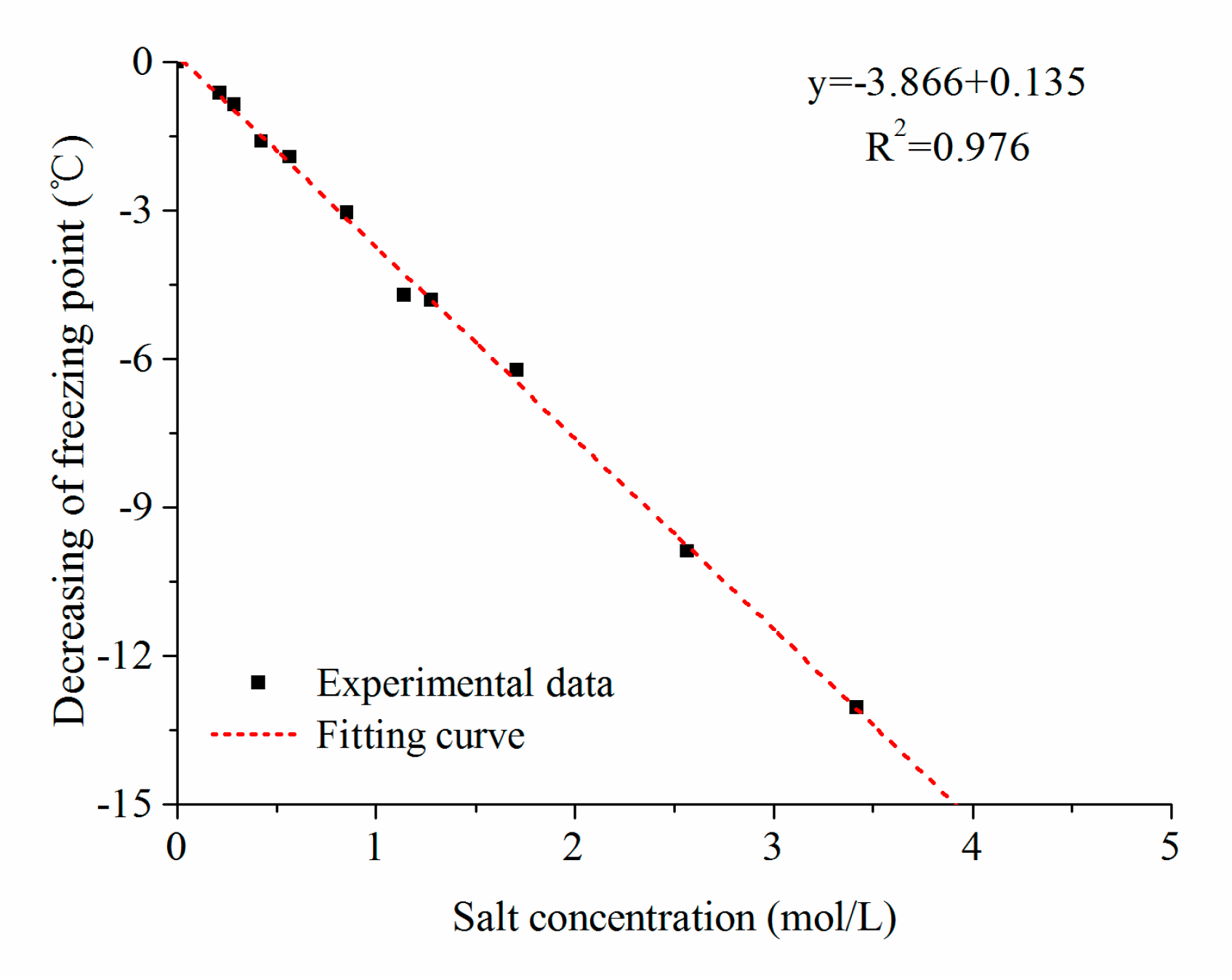
Water Free Full Text Investigation Into Freezing Point Depression In Soil Caused By Nacl Solution Html
Freezing Point Depression And Boiling

Freezing Point Depression Examples In Everyday Life Studiousguy

Unit 4 Solutions Lesson 6 C Perform A Lab To Demonstrate Freezing Point Depression And Boiling Point Elevation C Explain Freezing Point Ppt Download

Molar Mass And Freezing Point Depression Lab Prep Ppt Download

Pdf Freezing Point Depression In Model Lennard Jones Solutions

Constitutional Supercooling And Freezing Point Depression A Planar Download Scientific Diagram

Freezing Point Depression Examples In Everyday Life Studiousguy

The Science Of Ice Cream Freezing Point Depression Foodcrumbles
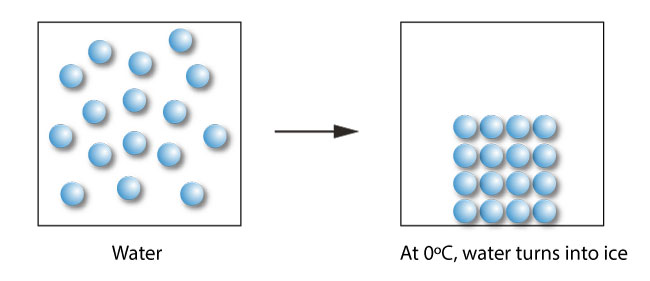
Freezing Point Depression Colligative Property

Freezing Point Depression Chemistry For Non Majors

Pdf Effects Of Freezing Point Depression On Molecular Weight Determination Of Hydrocarbon Mixtures

Boiling Point Elevation And Freezing Point Depression Example 3 Video Chemistry Ck 12 Foundation
Comments
Post a Comment